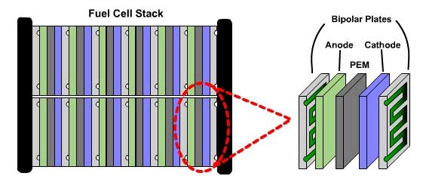
A fuel cell produces electricity through a chemical reaction, but without combustion. It converts hydrogen and oxygen into water, and in the process also creates electricity. It’s an electro-chemical energy conversion device that produces electricity, water, and heat.
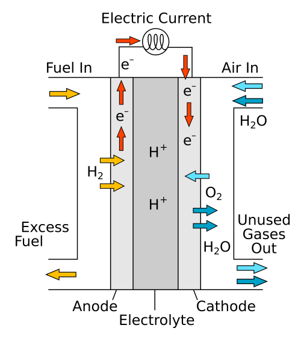
The reactions that produce electricity happen at the electrodes. Every fuel cell has two electrodes, one positive, called the anode, and one negative, called the cathode. These are separated by an electrolyte barrier. Fuel goes to the anode side, while oxygen (or just air) goes to the cathode side. When both of these chemicals hit the electrolyte barrier, they react, split off their electrons, and create an electric current. A chemical catalyst speeds up the reactions here.

Fuel cells operate much like a battery, except they don’t require electrical recharging. A battery stores all of its chemicals inside and coverts the chemicals into electricity. Once those chemicals run out, the battery dies. A fuel cell, on the other hand, receives the chemicals it uses from the outside; therefore, it won’t run out. Fuel cells can generate power almost indefinitely, as long as they have fuel to use.